Software Development in Regulated Environments
How to survive the FDA
Dr. Sasha Göbbels
https://shrtr.name/sire/Overview
- Short (!) about me
- What are we talking about?
- Medical Systems - GAMP 5
- Practical Approach
- References
About me
|
|
What are we talking about?

Paul McNulty
Former US Deputy Attorney General |
If you think compliance is expensive - try non-compliance. |
What is regulation?
Regulation is the management of complex systems according to a set of rules and trends.
Source: Wikipedia
- Regulation is dependent of national law and maybe EU
- Regulatory norms and standards are set by engineering institutions or lawmakers
- Sometimes national institutions adopt regulations from other countries
- In software development: your customer will insist on a validated product/process
Examples for regulated industries
Medical: FDA, BfArM
Financial: FINRA, BAFIN
Aerospace: FAA, EASA
Financial: FINRA, BAFIN
Aerospace: FAA, EASA
Regulation in industry
- Not every part of an industry is regulated
- Regulation applies where damage can be caused
- Regulation always consists of 2 parts:
- Where does regulation apply?
- What are the rules if it applies?
In this talk
- We'll talk about medical systems
- They are among the most tightly regulated
- Please ask me about other industries
- This is not a GMP training, just an overview!
Medical Systems - GAMP 5

A Glossary
GMP - Good Manufacturing Practice
GAMP - Good Automated Manufacturing Practice
SOP - Standard Operating Procedure
SME - Subject Matter Expert
QMS - Quality Management System(s)
ISPE - Int. Society for Pharmaceutical Engineering
FMEA - Failure Mode and Effects Analysis
GAMP - Good Automated Manufacturing Practice
SOP - Standard Operating Procedure
SME - Subject Matter Expert
QMS - Quality Management System(s)
ISPE - Int. Society for Pharmaceutical Engineering
FMEA - Failure Mode and Effects Analysis
Examples for medical systems requirements
- DIN EN ISO 13485:2016-08 - QMS General requirements
- FDA 21 CFR Part 211.68 - Automatic, mechanical & electronic equipment
- FDA 'Annex 11' - Computerized systems
- GAMP 5 - A Risk-Based Approach to Compliant GxP Computerized Systems
Definition of a computerized system
A computerized system is a combination of software and hardware components that together perform certain functions.
Source: FDA 21 CFR Part 211.68, Annex 11
Definition of validation
Documented evidence that a specific process continuously produces a product that meets predefined specifications and quality characteristics with a high degree of certainty.
Source: FDA 21 CFR Part 211.68
Focus on GAMP 5
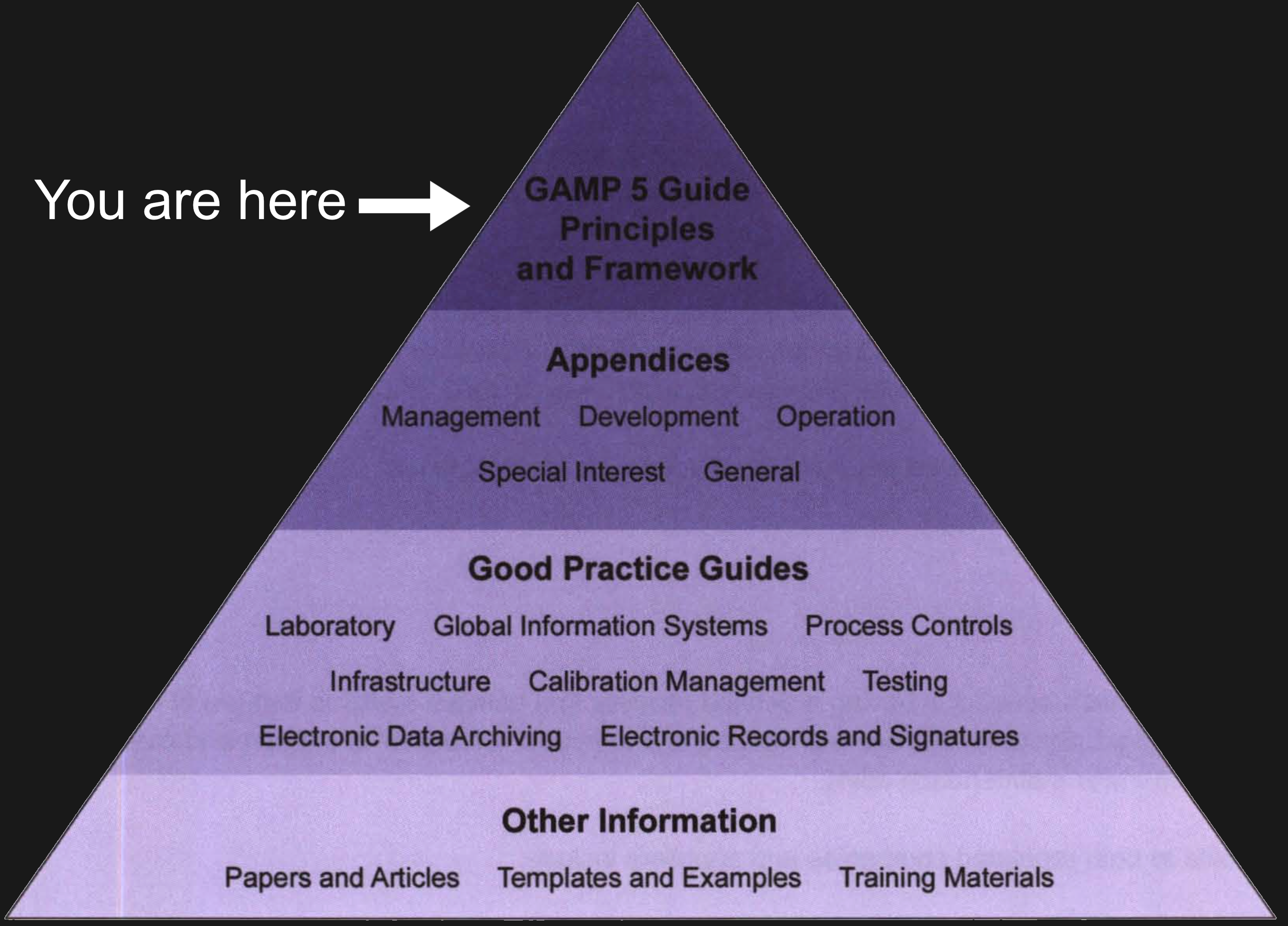
Some remarks on GAMP 5
- GAMP 5 is an industry standard, not a law
- In computerized systems GAMP 5 is today the standard
- Validation ≡ qualification
- Validation also includes user training and environment validation
- The process includes all phases of a product, even the decommissioning/shutdown
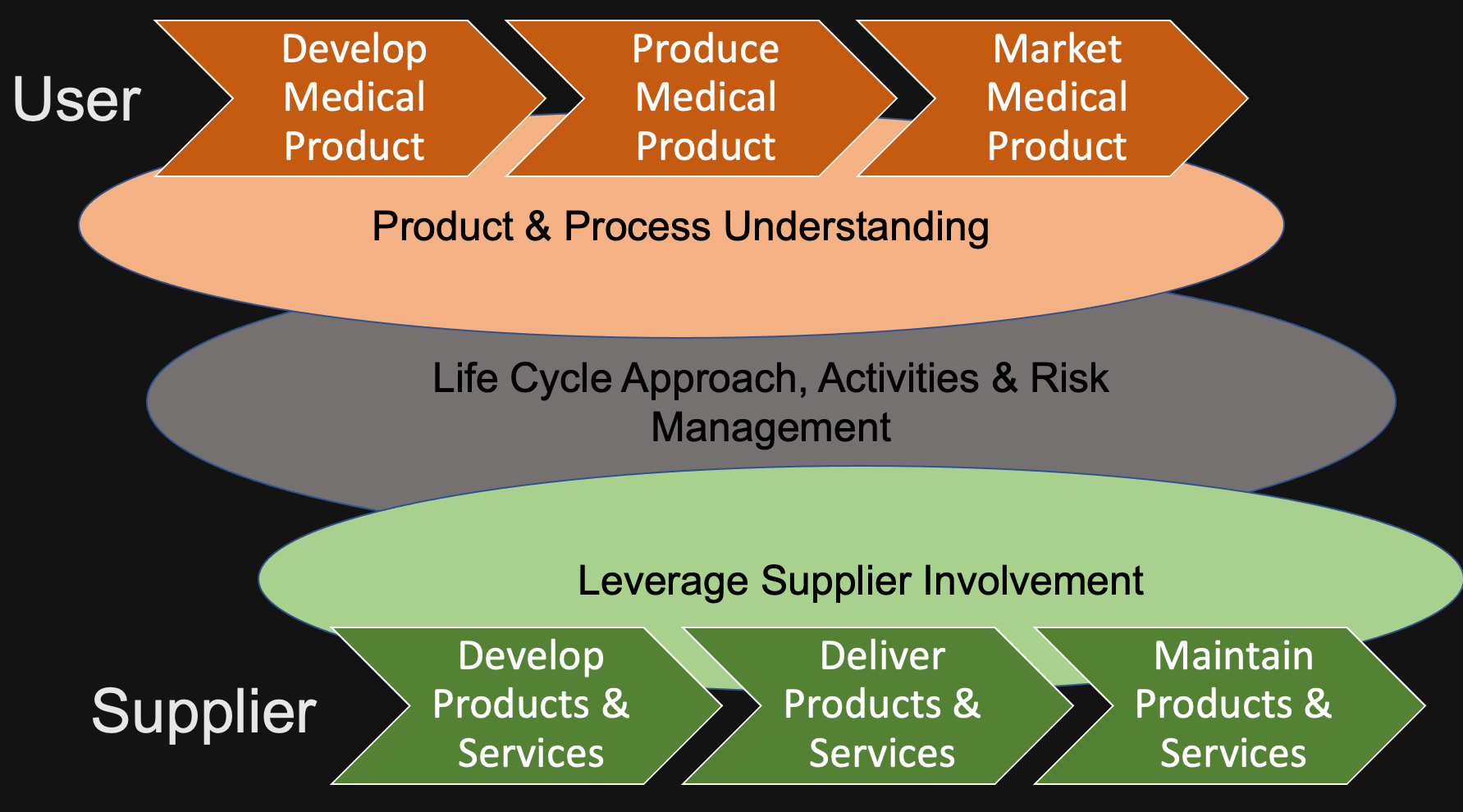
Five key concepts
- Product and process understanding
- Life cycle approach within a QMS
- Scaleable life cycle activities
- Science based quality risk management
- Leveraging supplier involvement
The Systems Process
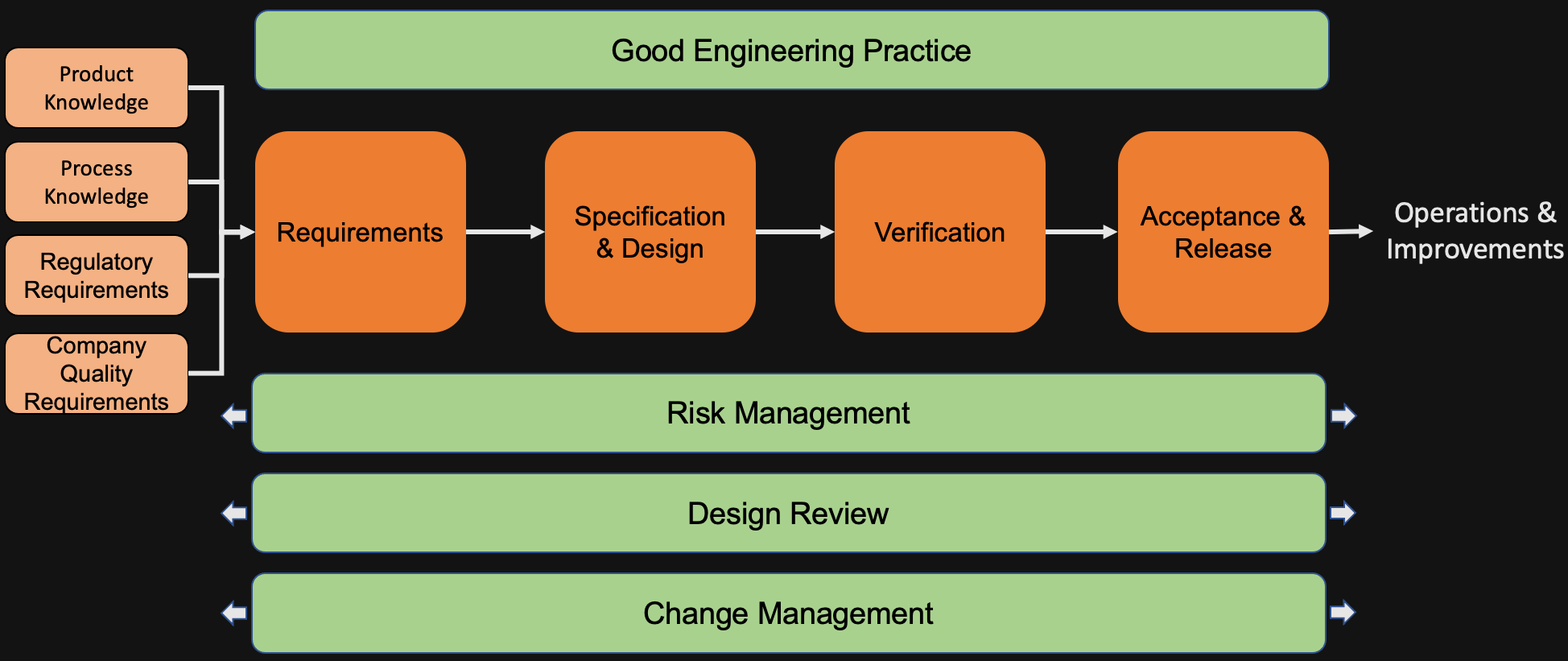
The Software Process
GAMP 5 categories
| Cat. | Name | Description | Examples |
|---|---|---|---|
| HW 1 | Std. comp. | Built in high quantities | Router, SPS |
| HW 2 | Cust. comp. | Indiv. hardware in low quantities | Indiv. control unit |
| SW 1 | Infrastructure | Shrink wrapped software | OS, Office, DBMS |
| SW 2 | Firmware | Not used any more, see 1 & 3 | OS of a switch |
| SW 3 | Non-configurable prod. | Comm. software that needs no config | Barcode scanner, temp. sensor |
| SW 4 | Configurable prod. | Std. software that needs config | SAP S4, LIMS, DMS |
| SW 5 | Custon applications | Custom built software | Everything else incl. VBA macros or interfaces |
Oh NO! It's a V!
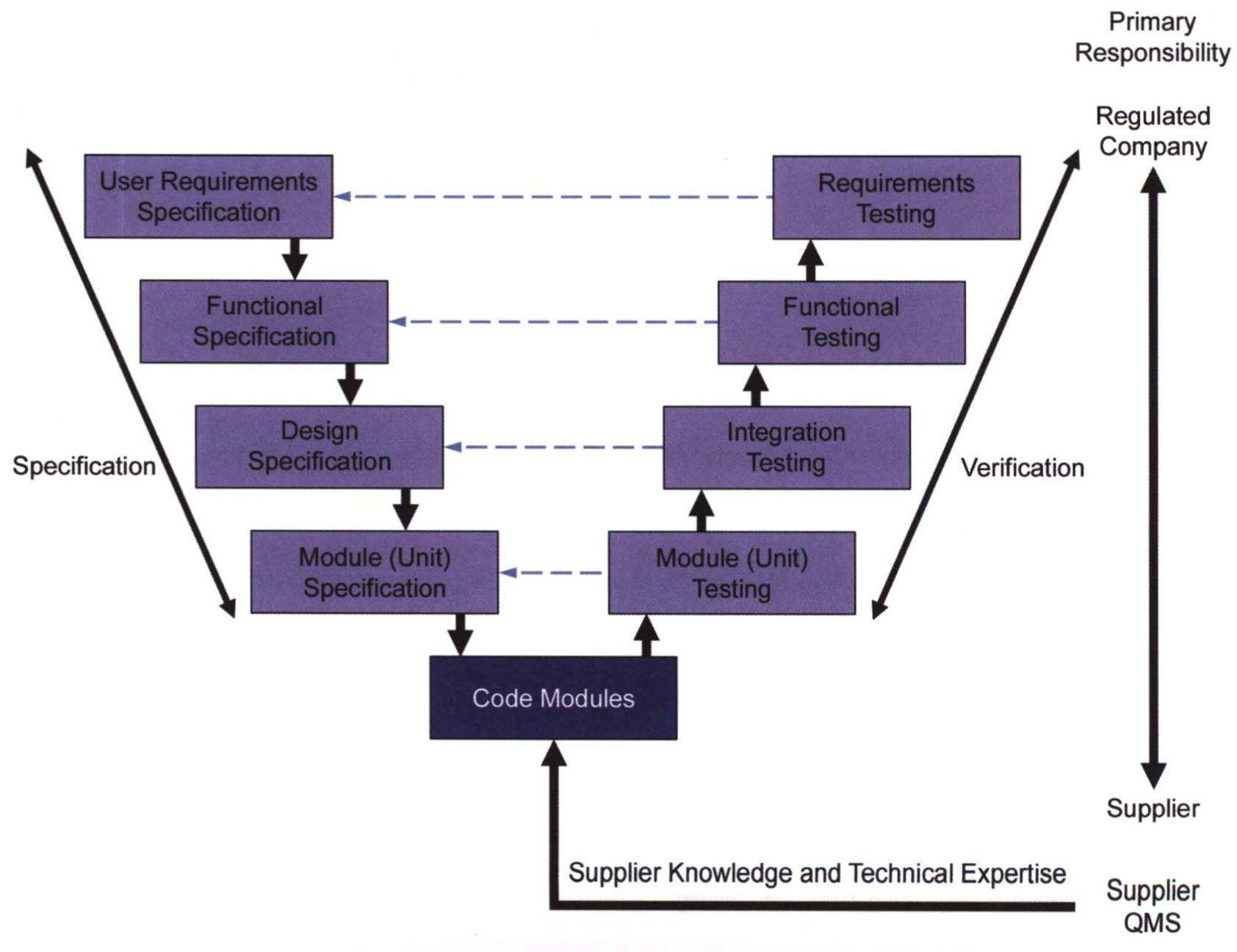
Who's afraid of the big V?
- The V model of GAMP 5 deals with the GAMP process, not the development process
- V should not strictly be seen as a temporal model
- You can specify components on the go while developing (yay, agile!)
Waterfall! It doesn't get better!
Design Control Process
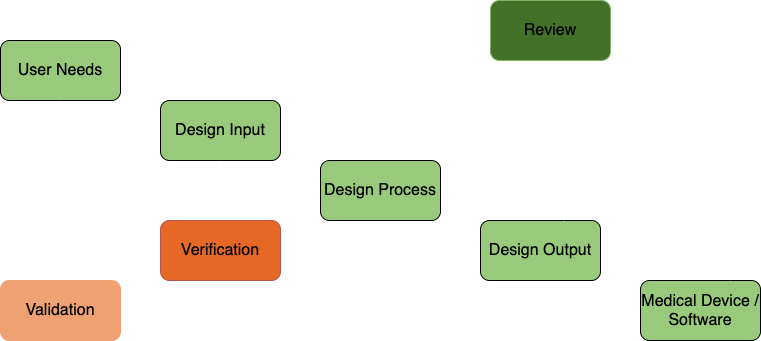
Design Control Nomenclature
| Input | Physical and perf. requirements |
|---|---|
| Output | Results of design effort at each design phase |
| Review | Documented, comprehensive, systematic examination of a design |
| Verification | Confirmation by examination that spec. requirements have been fulfilled |
| Validation | Confirmation by exam. that spec. requ. have been fulfilled for spec. use |
Practical Approach

So what do we do?
- Agile development in a regulated environment?
- Categorize hardware/software in GAMP category
- Requirements management (can be partially deferred)
- Risk management over the whole process (FMEA e.g.)
- Quality management
- Development itself
- Traceability over the whole process
Agile development in a regulated environment?
- Assess that you have the right people in the team
- Assess that the organization is willing to adapt its processes
- Start with a minor project
- Don't choose the most pressing important project
- Expand experience, knowledge and acceptance
Obstacles on the way to agile development
- The auditors/validators are not the problem
- The standards are not the problem
- Middle management adhering to established processes is
- Pay attention to systems theory!
Requirements management
Standard procedure, will not go into details.
Risk Management 1
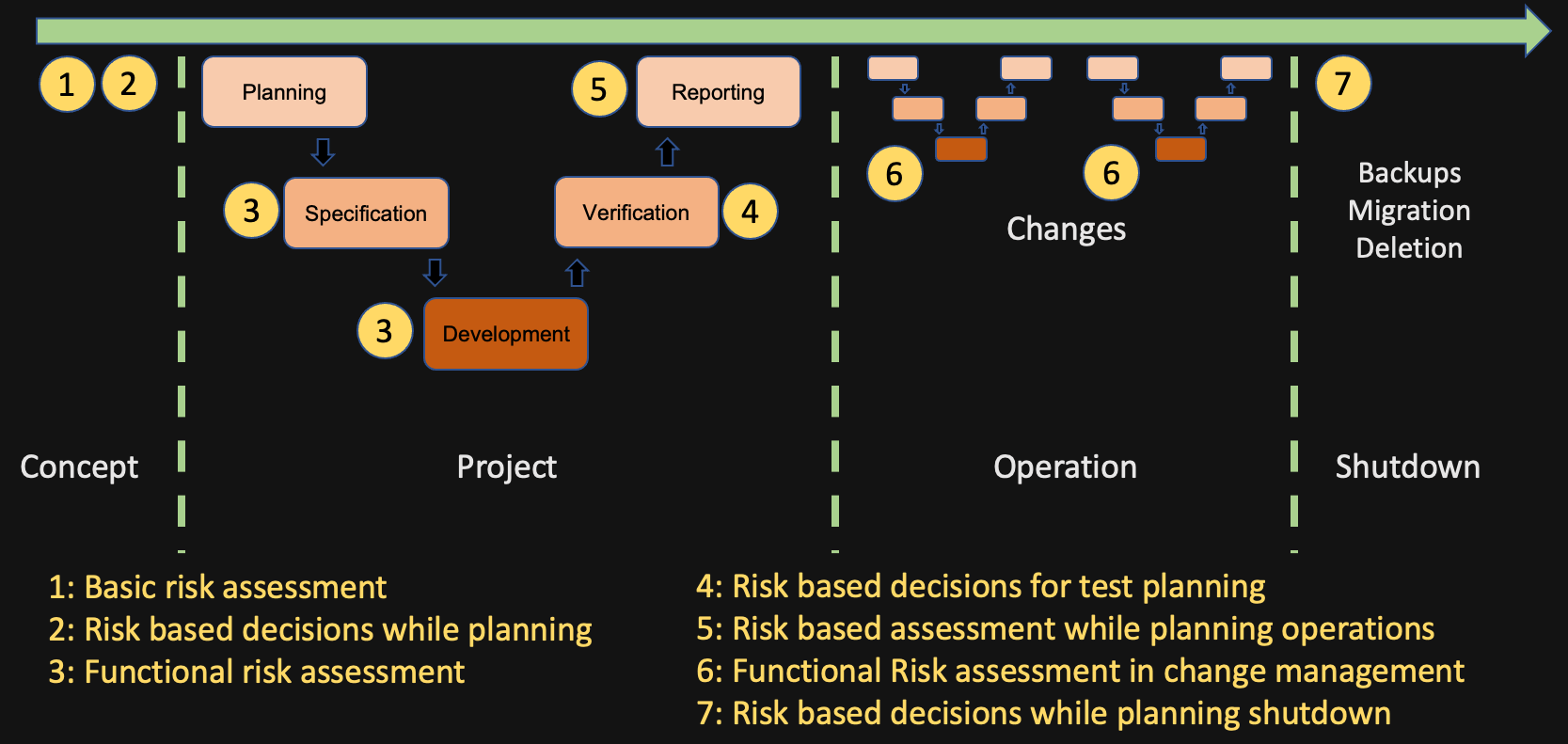
Risk Management 2
How to do risk management
|
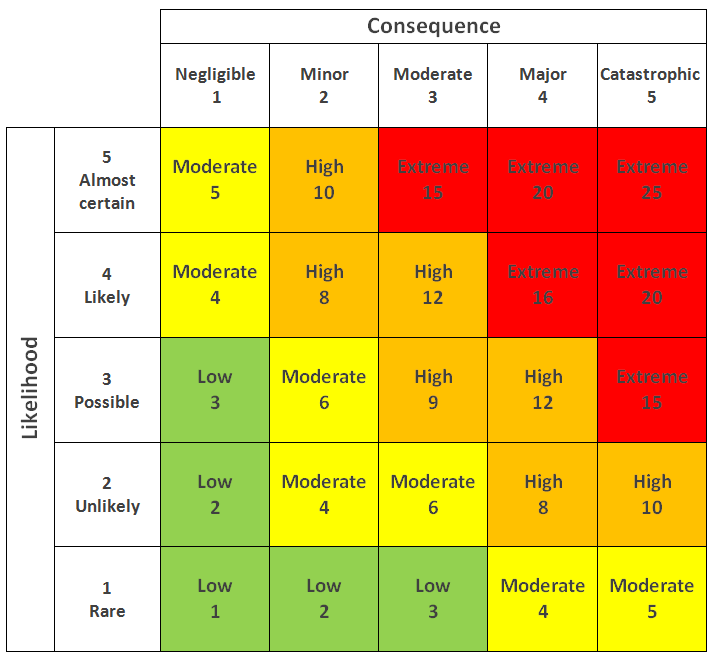 |
Risk Register Overview
- There's specialized software
- There are plugins for tools like Jira
- In its simplest form two tables for risks and mitigations
- It's an n:m relation - several risks can have the same mitigation(s) and one risk can have several mitigations
Risk Register Table
Possible columns:- Risk ID
- Description
- Knowledgeable persons
- Topic (project risk, technical risk, business risk)
- Consequence, likelihood & rating of risk
- Link to mitigation (mitigation ID)
- Consequence, likelihood & rating after mitigation
Mitigation Table
Possible columns:- Mitigation ID
- Description of mitigation
- Ticket number in tracker
- Mitigation owner
- State of implementation
Traceability Matrix
Possible columns:- User Specification ID
- Design Specification ID
- Risk ID (not every row will a risk associated)
- Installation Specification ID
- Operation Qualification ID
- Performance Qualification ID
- Requirement satisfied?
- Rationale / Mitigation
Change Management
Standard procedure, will not go into details.
Thank you!
Questions or Additions?

References
- AAMI TIR45: 2012 Technical Information Report Guidance on the use of AGILE practices in the development of medical device software
- Griffin Jones, "What is good evidence"
- Griffin Jones, "Surviving an FDA Audit"
- Griffin Jones, "Regulated Software Testing"
- STAREAST 2015 Interview with Griffin Jones on Regulated Software Testing

